Warm fronts are characterized as being low pressure systems because warm air is being lifted to create precipitation events. Pressure stays the same.
This leads to a greater pressure.
. This in terms increases the pressure of the system. What happens to pressure if temperature increases. A change in volume.
Are you referring to gasesIn gasesif the temperature increases then the pressure would also increase. Pressure is created by the number of collisions that occur between the molecules and the surface of container. By increasing the number of collisions this will increase the pressure in the container.
For example when the pressure increases then the temperature also increases. Temperature affects the lows and highs of air pressure but air pressure can also bring in higher or lower temperatures. PV nRT Pressure Volume Temperature Moles We know that temperature is proportional to the average kinetic energy of a sample of gas.
So greater momentum therefore greater force when they collide with the container walls so the pressure increases. If you heat a gas you give the molecules more energy so they move faster. If temperature increases what happens to pressure.
Also isobutane is combustible so incineration could cause the can to explode. Why does pressure increase when temperature increases Posted on April 23 2021 by admin Frequently asked questions. KE ave 23RT As the temperature increases the average kinetic energy increases as does the velocity of the gas.
This means more impacts on the walls of the container and an increase in the pressure. This causes the force on the walls of the container to. So the first response of the free floating ideal gas will be to become more excited.
In a direct relationship one variable follows the same change when it comes to increasing and decreasing. Remain the same d. 31 How do you find pressure when temperature increases.
As molecules move faster the number of collisions that will occur will increase. Temperature is directly related to kinetic energy. Faster moving particles will collide with the container walls more frequently and with greater force.
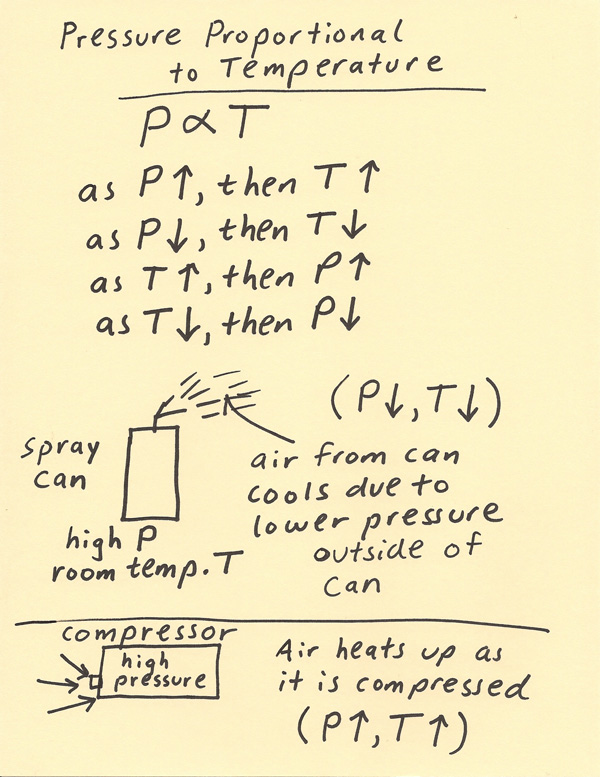
As the temperature increases the kinetic energy of the gas molecules increases the molecules move faster further away from each other. On a weather map warm fronts are labelled as curved red. The first is that of a spray can.
The temperature of the gas is proportional to the average kinetic energy of the gas molecules. The answer is D. Both gases have the same densities.
A The can contains an amount of isobutane gas at a constant volume so if the temperature is increased by heating the pressure will increase proportionately. Thus these particles collide with the walls of the container more frequently and forcefully. At constant temperature if the pressure of a gas increases the volume will increase decrease remain the same not enough information cant tell 3 Question 2 1 point Saved At constant volume if the moles and temperature of a gas increase the pressure will 6 increase decrease remain the same not enough information cant tell.
This will create a slow pressure wave and the volume will expand in response to the increased molecular collisions the pressure. A change in temperature. Conversely if you cool the molecules down they will slow and the pressure will be decreased.
30 What effect does the increase in temperature have on pressure in this period of time for heavier particles. If the temperature remains constant and the volume that gas occupies increases the pressure will ____. High temperature could lead to high pressure causing the can to burst.
32 How does temperature and pressure change with depth in the earth. The pressure will increase if the volume remains the same. The speed and movement of molecules is what determines air pressure.
When temperature increases the particles will have a greater average kinetic energy and a greater average speed. The volume of gas varies inversely with the absolute pressure provided the temperature remains constant. This is called _____.
Molecules in both gases have the same average kinetic energies. All collisions of the molecules are elastic. The volume of a sample of oxygen is 3000 mL when the pressure is.
Both gases have the same densities. When the temperature of a particular system is increased the molecules in the gas move faster exerting a greater pressure on the wall of the gas container. After the passage of the warm front the temperature increases since temperatures behind the front are warmer than temperatures ahead of the front.
Cannot be determined for the information given. If the temperature of the system is decreased the pressure goes down. Proportionality of Temperature and Pressure Atmospheric pressure and temperature are proportional meaning that when the temperature increases air pressure increases and.
The proportionality constant is 23R and R is the gas constant with a value of 008206 L atm K-1 mol-1 or 83145 J K-1 mol-1. The particles moving faster collide with the container walls frequently with greater force. There are 2 ways pressure increases.
This causes the force on the walls of the container to increase and so the pressure increases. There are two examples that demonstrate this principle. If the temperature in the container is increased this will cause the molecules to move faster.
When the pressure decreases then the temperature decreases. What happens to the pressure if the temperature is increased. Similarly a decrease in temperature will cause the molecules to have less kinetic.
Relationship Between Pressure and Temperature.

Factors Affecting Gas Pressure Ck 12 Foundation

0 Comments